
Solved Name Lab Partner Lewis Structure (ethanol,
Several intermolecular bonds exist and these include: 1) Hydrogen bonds - the strongest; formed between polar molecules 2) London dispersion forces (LDF) - the weakest; formed between nonpolar molecules 3) Ion-dipole bonds - formed between polar and ionic compounds Personally CH4 should not be included in the list because it is naturally in the.
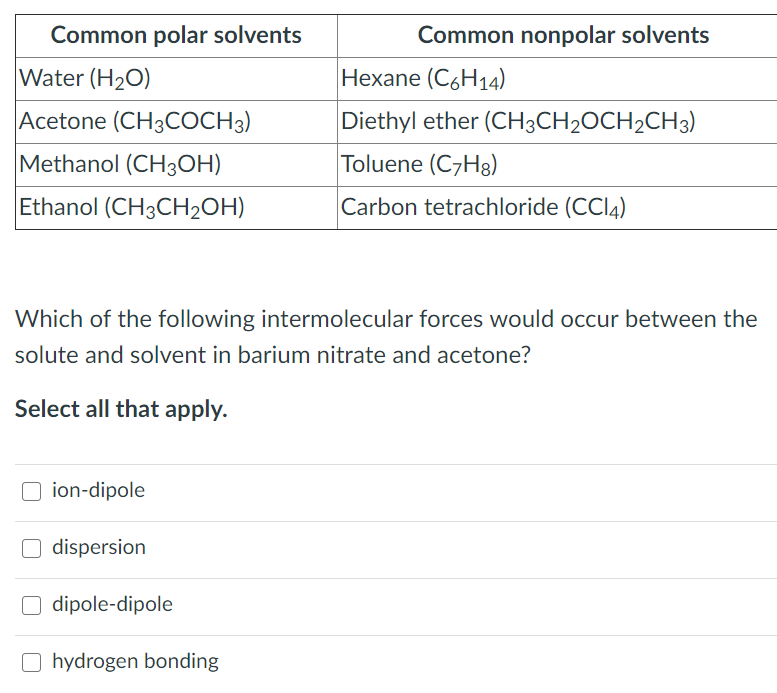
Solved Common polar solvents Water (H20) Acetone (CH3COCH3)
Learn to determine if CH3OH (Methanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.
MakeTheBrainHappy Is CH2O Polar or Nonpolar?
Chemistry Chemistry questions and answers 4. Compound: CH3CH2OH (Ethanol) Lewis Structure 3-D Structure Skeletal Structure Functional Group (s) Present: Central Atom (s) Geometry: Polar or Non-Polar 5.

Is CH3Cl Polar or Nonpolar? YouTube
This answer is: Add your answer: Earn + 20 pts Q: Is ch3ch2oh polar or nonpolar Write your answer. Submit Still have questions? Find more answers Ask your question Continue Learning about.

is ch3nh2 polar
CH 3 CH 2 OH represents the chemical formula for ethanol, aka ethyl alcohol, a primary alcohol commonly found in alcoholic beverages, cosmetics, perfumes, and spirits. Ethyl alcohol is a clear, colorless liquid (molar mass = 46.07 g/mol) often used as a solvent for various industrial applications.

Is CH3CH2OH Polar or Nonpolar? Polarity of Ethanol
Steel, an alloy of iron and carbon and small amounts of other metals, is an example of a solid solution. Table 6.3.1 6.3. 1 lists some common types of solutions, with examples of each. A solution is made by dissolving 1.00 g of sucrose ( C12H22O11 C 12 H 22 O 11) in 100.0 g of liquid water.

draw the structure of ethanol molecule toctocquienes
H H ** ** ** H : C : C : O : H ** ** ** H H Q3. Give the electron pair and molecular geometries for each central atom. Q4. Give the bond polarity for each type of bond in the molecule. = There are two types of bonds in ethanol. The C-H bonds are nonpolar covalent. Q5. Is your molecule polar or nonpolar ? = Ethanol is both polar and nonpolar. Q6.

Is oxygen gas polar or nonpolar? Gek Buzz
The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity.

POCl3 Lewis Structure (Phosphoryl Chloride) Lewis, Math, Molecules
Is the molecule C H 3 C H 2 O H polar or nonpolar? Explain. Polar and Non-Polar Molecules The electronegative difference between the atoms gives rise to polarity in the molecule. An example.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Is O2 Polar Or Nonpolar?
Expert-verified. Polarity of ethanol: ethanol is a polar molecule ( with dipole moment= 1.69)due to presence of hydro.. Name: Lab Partner: Lewis Structure (ethanol, CH3CH2OH) Date: Sketch - indicate polar bonds C - H ( H H Polar Bonds (Yes or No)? bo nd blo H 3c are nonPc lar H-o, c-O ave Polav Molecular Geometry at Carbon Atoms Molecular.

Best Overview Is CH3F Polar or Nonpolar Science Education and Tutorials
Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.

Lewis Structure For Ethanol
Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct.

Is H2O polar or nonpolar and why? YouTube
Polar vs Non-Polar molecules . As indicated in Table 2.6, the nature of molecular polarity determines the types of force(s) applied to a certain substance. So here we will have discussions about how to tell whether a molecule is polar or non-polar. The polarity of the compound can be determined by its formula and shape.

science chemistry miscibility Fundamental Photographs The Art of
5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions.

Is Ch3Ch2Oh Polar Or Nonpolar? Understanding The Nature Of This
Ethanol (CH3CH2OH) is an extremely polar chemical compound. Each CH3CH2OH molecule comprises two C-atoms, six H-atoms, and an O-atom. A small electronegativity difference (0.35 units) is present between a carbon and a hydrogen atom in each C-H bond.